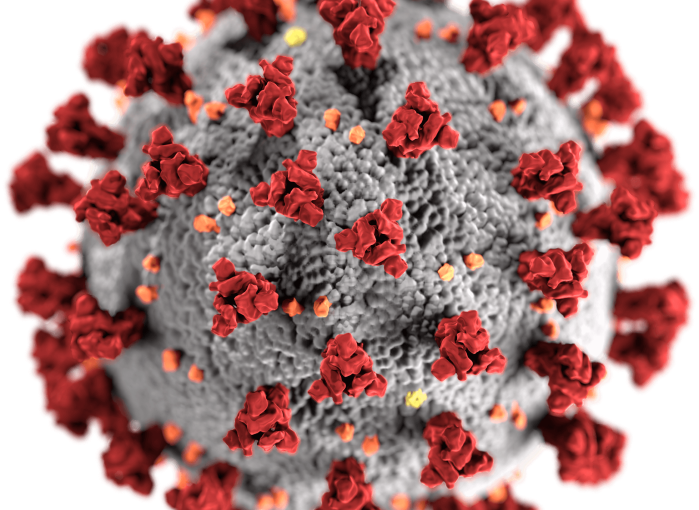
Kateryna Kon/Shutterstock
With omicron having rapidly driven up COVID infections, attention is once again focusing on antibodies, and reasonably so. They play a critical role in fighting off viruses and are important for preventing the coronavirus infecting our cells. This is why some countries have mounted booster campaigns in response to recent COVID surges – to top antibody levels up.
But there’s a problem. COVID antibodies don’t persist that well – hence the desire for boosters. Indeed, while these extra jabs maintain good protection against severe COVID, it’s estimated that people receiving a third dose of the Pfizer vaccine will see their protection against developing COVID symptoms (of any degree) drop from 75% to 45% over the ten weeks following their booster. Scientists have questioned whether topping up antibodies, only to see them soon fade away, is sustainable.
If we want to develop lasting immunity to COVID, it’s perhaps time to look again at our wider immune response. Antibodies are just one part of our intricate and intertwined immune system. Specifically, it’s maybe time we focused on T cells.
How different immune cells work
When the body is infected, say with a virus, it responds by producing white blood cells called lymphocytes. The main types are B cells, which make antibodies, and T cells, which either support B cell antibody production or act as killer cells to destroy the virus. Some T cells and B cells also become long-lasting memory cells that know what to do if they meet the same infection again.
B cells and T cells “see” the virus in different ways. Generally speaking, B cells recognise shapes on the outside of the virus, creating antibodies that will lock on to those (a bit like two jigsaw pieces that match). T cells instead recognise bits of the amino acids that build the virus, including bits that might normally be found inside it.
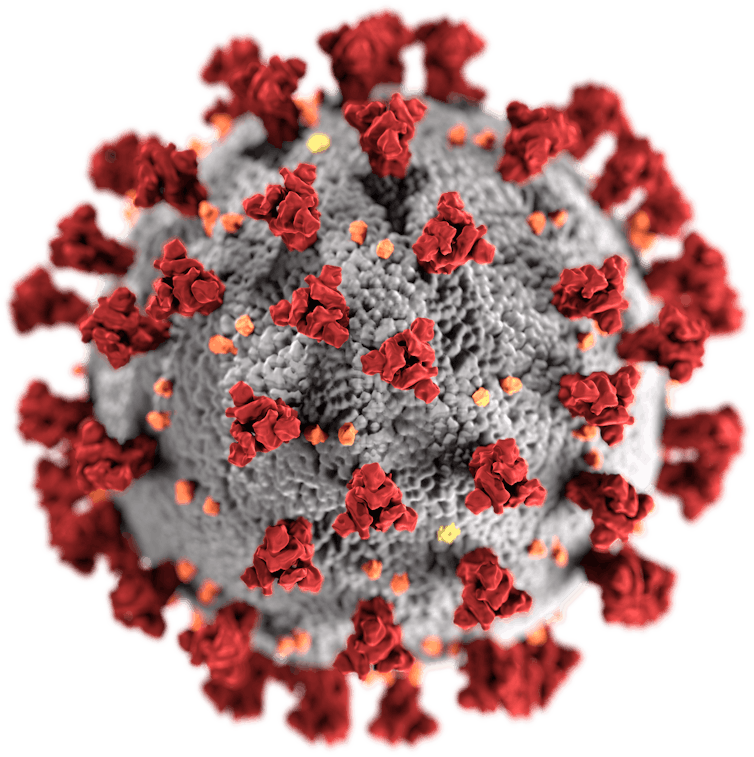
US CDC/Wikimedia Commons
Every virus has lots of unique features, both inside and out. A person’s immune response can end up making a variety of T cells and B cells that between them target a whole range of these features. This is sometimes called “breadth of response”. A good breadth of response has lots of different lymphocytes that see different parts of the virus, making it very tricky for the virus to completely hide from them.
Omicron worried many researchers because a key part of its external structure that’s targeted by antibodies – the spike protein (in red in the picture above) – is heavily mutated, lessening the ability of antibodies to bind to the virus and neutralise it. However, because T cells focus on other parts of the virus, such mutations might not stop them from identifying it.
Indeed, early data, which is still awaiting review, suggests this is the case. This is reassuring, because the virus’s spike protein has changed a lot during the pandemic, suggesting that it could always be mutating away from the reach of antibodies. T cells, though, should be less susceptible to viral mutation. T cells designed to fight COVID also appear to be much longer lasting in the human body than antibodies.
But do T cells have a strong effect?
We already know a lot about the critical role of T cells in other viral infections. This knowledge suggests that, against COVID, a good T cell response is not only needed to help B cells produce antibodies but should also create killer T cells that can broadly recognise the coronavirus, protecting against multiple variants.
Evidence directly on COVID and T cells is still being gathered. However, it’s gradually becoming clearer that T cells do seem to play a big role in COVID.
Research has shown that generating broadly reactive T cells that recognise a range of viral features is associated with a strong response against the disease. Generating good amounts of broadly reactive killer T cells in particular seems to make COVID less severe.
Conversely, a poor T cell response is associated with worse outcomes for patients. Indeed, some people who have had severe COVID have been found to have persistent defects in their T cell response.
A common feature of many of the studies demonstrating the effectiveness of T cells in COVID is the need for a wide breadth of response – having T cells (and B cells) that recognise multiple features of the virus. It’s thought that this could be the key to experiencing milder disease.
This breadth might even extend beyond this coronavirus specifically. The COVID virus is a betacoronavirus, and there are several betacoronaviruses that already infect us, including ones that cause the common cold. Shared features between these cold-causing viruses and COVID may mean that T cells we already had against the cold are protecting us against COVID now. Evidence for this in both adults and children is being uncovered.
What does this mean for vaccines?
Many of the vaccines designed to date – including Moderna’s, Pfizer’s and AstraZeneca’s – have focused on just one major target on the coronavirus: its spike protein. These vaccines have been tremendously effective at generating antibodies. They also stimulate a T cell response to the spike.
But now that we understand more about the role of T cells, the importance of having a broad T cell response, and the issue of antibodies waning, perhaps we should consider refocusing our vaccine strategies on generating T cells and on targeting more than just one protein.
Work is moving in this direction. Early trials of vaccines that can trigger much more broadly reactive helper and killer T cell responses have been completed, and several other T cell vaccines are also entering trials.
These T cell vaccines may be the key to boosting existing immunity and generating long-lived protection against severe disease from a whole range of COVID variants. If so, they would be a huge part of the world living more safely with COVID.
![]()
Sheena Cruickshank does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Source: TheConversation